Letter from President Jarrell: Take Pride in UMB: Vaccine News
December 09, 2020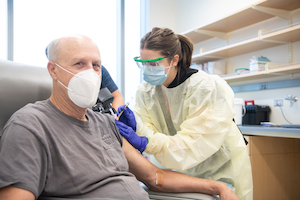
Dear UMB Community,
This year has been one for the history books. By now, everyone is gearing up for a tough winter with COVID-19 numbers on the rise. Many of us have our work-from-home routines, we consistently adjust to changes with family and school obligations, and we are creating very different holiday practices. Feelings range from isolation and loneliness to wishing for quiet and somewhere to go. Reflecting on the year behind us, the two overwhelming feelings I have about the University of Maryland, Baltimore (UMB) are pride and hope.
You should be extremely proud to be part of UMB. When people can finally gather safely in this country, it will be in no small part because of the work of this institution. For more than four decades, our researchers in the Center for Vaccine Development and Global Health (CVD) at the School of Medicine have worked domestically and internationally to develop, test, and deploy vaccines. Our methods have led to incredible new and improved ways to diagnose, prevent, treat, control, and eradicate some of the world’s most serious diseases, and now we can add COVID-19 to that list.
Matthew Frieman, PhD, associate professor of microbiology and immunology at the School of Medicine, has focused his career on studying coronaviruses. In February, Dr. Frieman’s lab was one of the first in the country to receive samples of COVID-19 from China so it could begin studying the virus and researching drugs to combat it. Kathleen Neuzil, MD, MPH, the Myron M. Levine, MD, DTPH, Professor in Vaccinology and director of CVD, has been at the center of the domestic and global responses to COVID-19. As the co-principal investigator of the Infectious Disease Clinical Research Consortium of the National Institutes of Health (NIH), she is part of the strategic team evaluating COVID-19 vaccines and therapeutics in the United States, and she is part of the study team that designed the nation’s first COVID-19 clinical vaccine trials.
Dr. Neuzil also serves as co-director of the federal COVID-19 Prevention Network and has critical advisory roles with the Centers for Disease Control and Prevention and the World Health Organization. I’m also proud that our faculty experts have played a key role in advising Gov. Larry Hogan and his cabinet, addressing concerns and mapping out COVID-19 strategies across Maryland. The School of Medicine’s Wilbur Chen, MD, MS, professor of medicine, and David Marcozzi, MD, associate professor of emergency medicine and assistant chief medical officer for acute care at the University of Maryland Medical Center, are members of the governor’s Coronavirus Response Team.
On the research front, in May our researchers became the first in the U.S. to begin testing experimental COVID-19 vaccine candidates developed by Pfizer and BioNTech. In addition, UMB participated in the Phase 3 clinical trial of an investigational COVID-19 vaccine co-developed by scientists at Moderna, Inc., and the National Institute of Allergy and Infectious Diseases, part of NIH. I volunteered as a subject for that trial. The success rates of these vaccine candidates have been impressive, and our vaccine experts continue with their research, gearing up for another Phase 3 vaccine trial and with plans for research into pediatric COVID-19 vaccines.
I’m also especially proud of the targeted outreach carried out to make sure that the vaccines are safe and effective for different participants. We have prioritized reaching communities most impacted by this virus, including those at risk of exposure, elderly populations, and those with underlying health conditions. For the Moderna trial, UMB was one of the top recruiters for the populations most impacted by COVID-19 in the United States. CVD partnered with community organizations such as CASA de Maryland in Hyattsville to reach a highly impacted Latino community. For the first time ever, CVD conducted its research with COVID-19 vaccines off campus and in the community. Researchers have set up a satellite site in Hyattsville, and they are vaccinating and screening individuals there at CASA headquarters. Participants in this community, a hotspot for COVID-19 and largely Latino, can participate in the CVD trial locally, without having to come to UMB’s campus. You can hear from some of the trial participants in their own words here.
Like everything else in 2020, we have adjusted to new ways of doing things. These vaccine trials have happened at an unprecedented speed — but there have been no shortcuts in the process. Hours upon hours, weeks upon weeks, and months upon months of almost uninterrupted work have brought us to this exciting point. Take pride in knowing that vaccines are a safe and effective way to combat this virus, and that this institution plays an indelible part of changing the trajectory of the pandemic.
My feeling of hope comes from knowing that even though the days feel dark right now and we are in a frightening time for COVID-19 rates, I know we will weather this challenge together. It fills me with pride to see how this community dedicated to improving the human condition and serving the public good is doing just that in record-breaking time with these incredible vaccine discoveries.
I hope that you will serve as a messenger in your community and family, sharing your confidence in science and discovery, that you will encourage others to feel pride in UMB and to feel safe in taking the vaccine when it becomes available. Please tune in to Thursday's Virtual Face to Face program to learn more and ask questions about advancements in COVID-19 vaccines with guest Dr. Neuzil, and feel free to invite family, friends and colleagues to watch as well.
Sincerely,
Bruce E. Jarrell, MD, FACS
President