Strategic Prototyping for Medical Device Success
May 06, 2025 Dustin Lee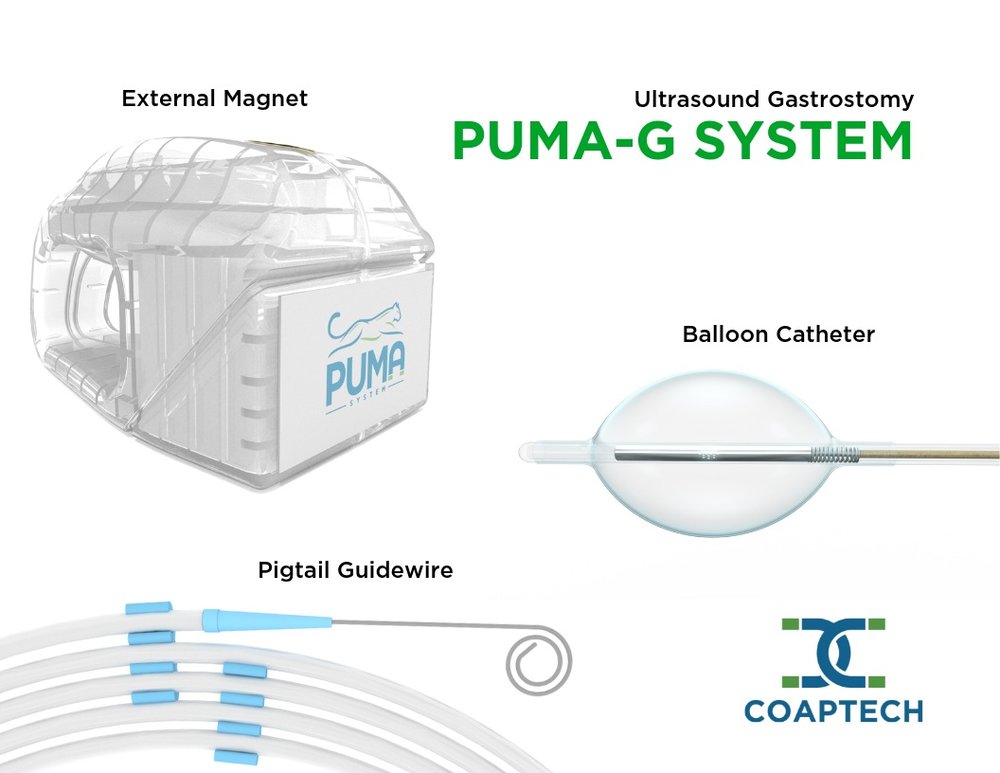
At the April Innovation @ UMB session, Root3 Labs shared its expert advice for successful academic medical device development.
Photo: CoapTech used feedback-informed prototyping to develop its PUMA-G System.
Medical devices face a multitude of unique challenges, including lengthy development cycles, high costs, complex regulatory frameworks, and diverse stakeholder interests. Academic medical device developers can be a critical partner, helping innovators navigate these challenges while supporting positive funding outcomes and ultimately maximizing their return on investment.
At the April Innovation @ UMB session, Root3 Labs, a device engineering and product development firm in the Baltimore region that works with academic medical device developers, shared its expert advice for successful academic medical device development.
- The Need for Strategic Innovation in Medical Devices: The challenges faced by the medical device industry demand a strategic approach. It can be helpful to continually monitor the maturity level of a technology using a measurement system such as the Technology Readiness Levels (TRL). TRL is a nine-stage framework ranging from initial observations to manufacturing and sales, and illustrates the extensive journey from concept to market.
- Strategic Discovery: A Foundation for Success: Strategic discovery is a process designed to maximize the return on investment by ensuring innovators pursue needs that are both viable and aligned with their capabilities. Needs should be validated before solution development. Validated needs are those that fit the innovator's focus, have a ready customer base, and are solvable with available resources. Veering from these needs can lead to wasted time and resources.
- The Strategic Discovery Process: The strategic discovery process involves a four-stage approach:
- Strategic Focus: Aligning stakeholders and defining project objectives
- Need Finding: Identifying potential needs through observation
- Need Definition: Interviews
- Ideation and evaluation: Market research
Each stage is supported by specific tools and outputs, including discovery meetings, ethnographic observation, need statements, low-fidelity prototyping, and ranked concepts.
- Defining and Prioritizing Needs: Need definition is a critical step, where each identified need is articulated as a problem, population, and desired outcome. This structured approach facilitates targeted innovation. Competitive analysis and understanding the scientific and market landscape further refine the needs. Finally, these needs are scored and ranked to determine which ones to pursue. The process culminates in the ideation and evaluation stage, where need specifications guide brainstorming and low-fidelity prototyping to validate early concepts.
- Prototyping Fundamentals: Prototyping is a crucial tool for learning, mitigating risk, and communication. There are several important factors for developing the appropriate prototype for each project. Innovators should consider resolution (low vs. high), quantity (many vs. one), and focus (works-like vs. looks-like). Often starting with low-resolution prototypes to address the biggest risks, followed by iterative refinement, is the best use of time and resources to learn and remain aligned with the validated needs. This is true for prototype progression across mechanical, electrical, and software domains.
- User Feedback and Case Studies: User feedback at all stages is important to the prototyping process. One example of feedback-informed prototyping using the principles of strategic discovery and prototyping is CoapTech. Steven Tropello, MD, applied this process to create a company around his idea for improving the insertion of feeding tubes while at the University of Maryland, Baltimore. The technology simplifies feeding tube insertion that has traditionally been complex, time-consuming, and uncomfortable for patients. It often involves multiple attempts, which can lead to complications such as tube misplacement, patient discomfort, and increased health care costs. The technology provides clinicians with a more precise and efficient way to insert feeding tubes, reducing the risk of complications and improving patient outcomes. The project involved several stages of development, including initial concept design, prototype construction, manufacturing process development, and iterative refinement based on testing and feedback.
A comprehensive framework for medical device innovation emphasizes the importance of front-end strategic planning and targeted prototyping. By emphasizing a needs-based approach and iterative development, the framework offers a valuable roadmap for researchers seeking to translate their ideas into impactful medical devices.